NANOSYSTEMS: PHYSICS, CHEMISTRY, MATHEMATICS, 2021, 12 (3), P. 283–290
Prooxidant potential of CeO2 nanoparticles towards hydrogen peroxide
M. M. Sozarukova – Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, Leninsky Prospect, 31, Moscow, 119991, Russia; s_madinam@bk.ru
E.V. Proskurnina – Research Centre for Medical Genetics, Moskvorechie St, 1, Moscow, 115522, Russia; proskurnina@gmail.com
V. K. Ivanov – Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, Leninsky Prospect, 31, Moscow, 119991, Russia; van@igic.ras.ru
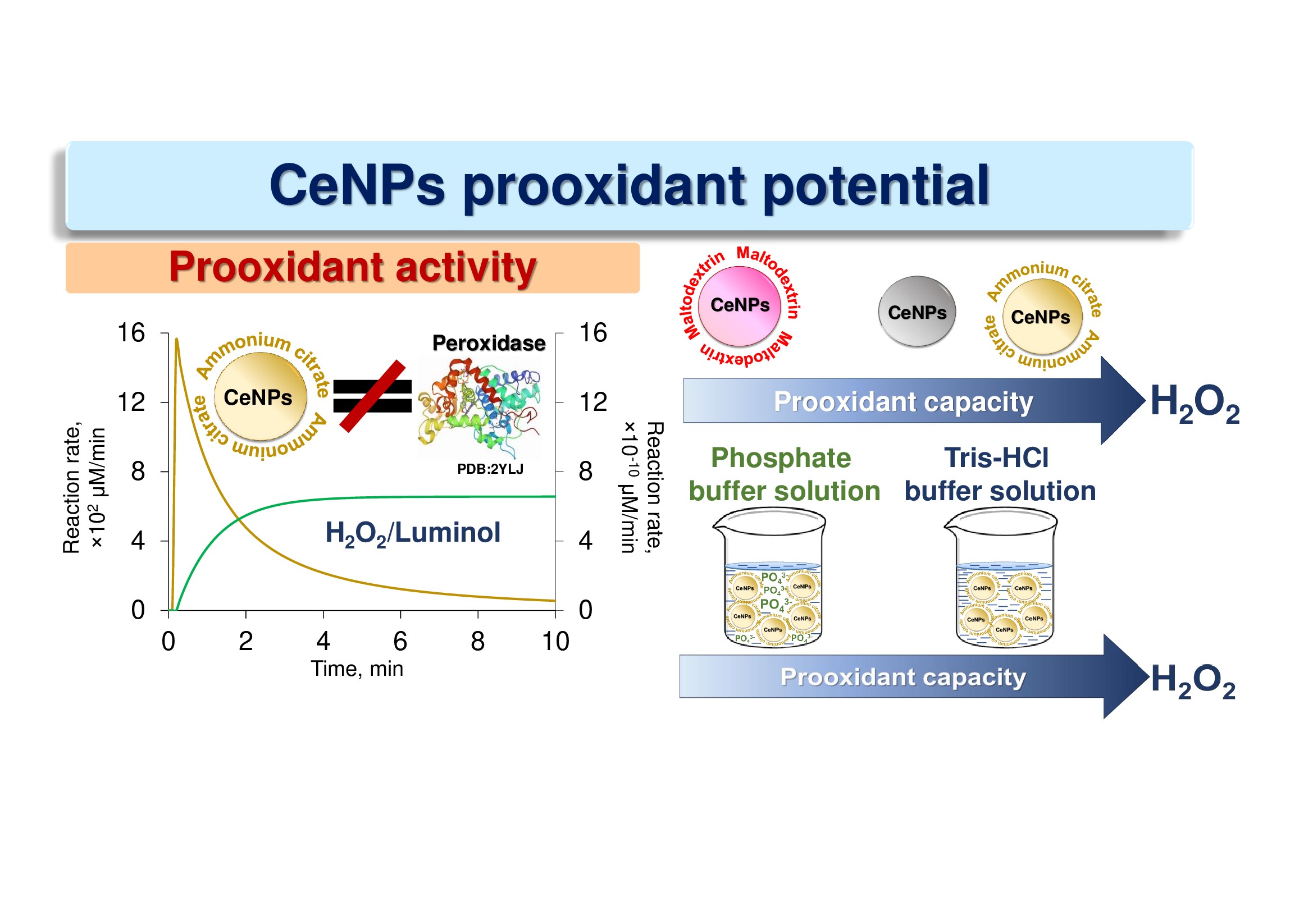
The multifaceted enzyme-like activity of CeO2 nanoparticles (CeNPs) expands the prospects for their potential biomedical applications. In this regard, there is a need for a comprehensive analysis of the redox behavior of CeO2 nanoparticles in relation to key molecules of free radical homeostasis. Here, the prooxidant potential of CeNPs towards H2O2 was investigated to elucidate both prooxidant capacity and prooxidant activity of CeNPs. To describe the kinetics in the luminol–H2O2 system at pH 8.5 upon the addition of citrate-stabilized CeO2 sol (3 nm), a numerical model of three reactions is proposed. The rate constants being a measure of prooxidant activity, were k1 = 9.0 ·104 μM-1min-1, k2 = 2.0 · 106 μM-1min-1, k3 = 2.9 ·105 μM-1min-1. The functionalization of CeO2 nanoparticles surface with ammonium citrate increases their prooxidant capacity by two-fold, while modification with maltodextrin decreases it by six-fold. It was shown that the prooxidant capacity of citrate-stabilized CeO2 sol in Tris-HCl is approximately four-fold higher than in phosphate buffer solution at pH 7.4.
Keywords: cerium dioxide nanoparticles, nanozymes, hydrogen peroxide, luminol, peroxidase, chemiluminescence, prooxidant, ammonium citrate, maltodextrin, mathematical modeling.
PACS 68.65.k, 81.20.n, 82.70.Dd, 87.85.Rs
DOI 10.17586/2220-8054-2021-12-3-283-290
[In Russian] М.М. Созарукова, Е.В. Проскурнина, В.К. Иванов
Прооксидантный потенциал наночастиц CeO2 по отношению к перекиси водорода
Многогранная ферментоподобная активность наночастиц CeO2 (CeNP) расширяет перспективы их потенциального биомедицинского применения. В связи с этим возникает необходимость комплексного анализа окислительно-восстановительного поведения наночастиц CeO2 по отношению к ключевым молекулам свободнорадикального гомеостаза. Здесь был исследован прооксидантный потенциал CeNP по отношению к H2O2, чтобы выяснить как прооксидантную способность, так и прооксидантную активность CeNP. Для описания кинетики в системе люминол–H2O2 при рН 8.5 при добавлении цитрат-стабилизированного золя CeO2 (3 нм) предложена численная модель трех реакций. Константы скорости, являющиеся мерой прооксидантной активности, составили k1 = 9.0·104 мкм-1мин-1, k2 = 2.0·106 мкм-1мин-1, k3 = 2.9·105 мкм-1мин-1. Функционализация поверхности наночастиц CeO2 цитратом аммония увеличивает их прооксидантную способность в два раза, а модификация мальтодекстрином снижает ее в шесть раз. Показано, что прооксидантная способность цитрат-стабилизированного золя CeO2 в Трис-HCl примерно в 4 раза выше, чем в фосфатно-буферном растворе при рН 7.4.
Ключевые слова: наночастицы диоксида церия, нанозимы, пероксид водорода, люминол, пероксидаза, хемилюминесценция, прооксидант, цитрат аммония, мальтодекстрин, математическое моделирование.