NANOSYSTEMS: PHYSICS, CHEMISTRY, MATHEMATICS, 2018, 9 (1), P. 41–45
Optimization of the solvent-exchange process for high-yield synthesis of aqueous fullerene dispersions
I.V. Mikheev – Chemistry Department, Lomonosov Moscow State University, Moscow; Analytical Centre of Lomonosov Moscow State University / Agilent Technologies Authorized Partner Laboratory, Moscow, Russia; mikheev.ivan@gmail.com
M. O. Pirogova – Chemistry Department, Lomonosov Moscow State University, Moscow, Russia; mariy7991@yandex.ru
T. A. Bolotnik – Chemistry Department, Lomonosov Moscow State University, Moscow; Analytical Centre of Lomonosov Moscow State University / Agilent Technologies Authorized Partner Laboratory, Moscow, Russia; timab@tut.by
D. S. Volkov – Chemistry Department, Lomonosov Moscow State University, Moscow; Analytical Centre of Lomonosov Moscow State University / Agilent Technologies Authorized Partner Laboratory, Moscow, Russia; dmsvolkov@gmail.com
M.V. Korobov – Chemistry Department, Lomonosov Moscow State University, Moscow, Russia; mkorobov49@gmail.com
M. A. Proskurnin – Chemistry Department, Lomonosov Moscow State University, Moscow; Analytical Centre of Lomonosov Moscow State University / Agilent Technologies Authorized Partner Laboratory, Moscow, Russia; proskurnin@gmail.com
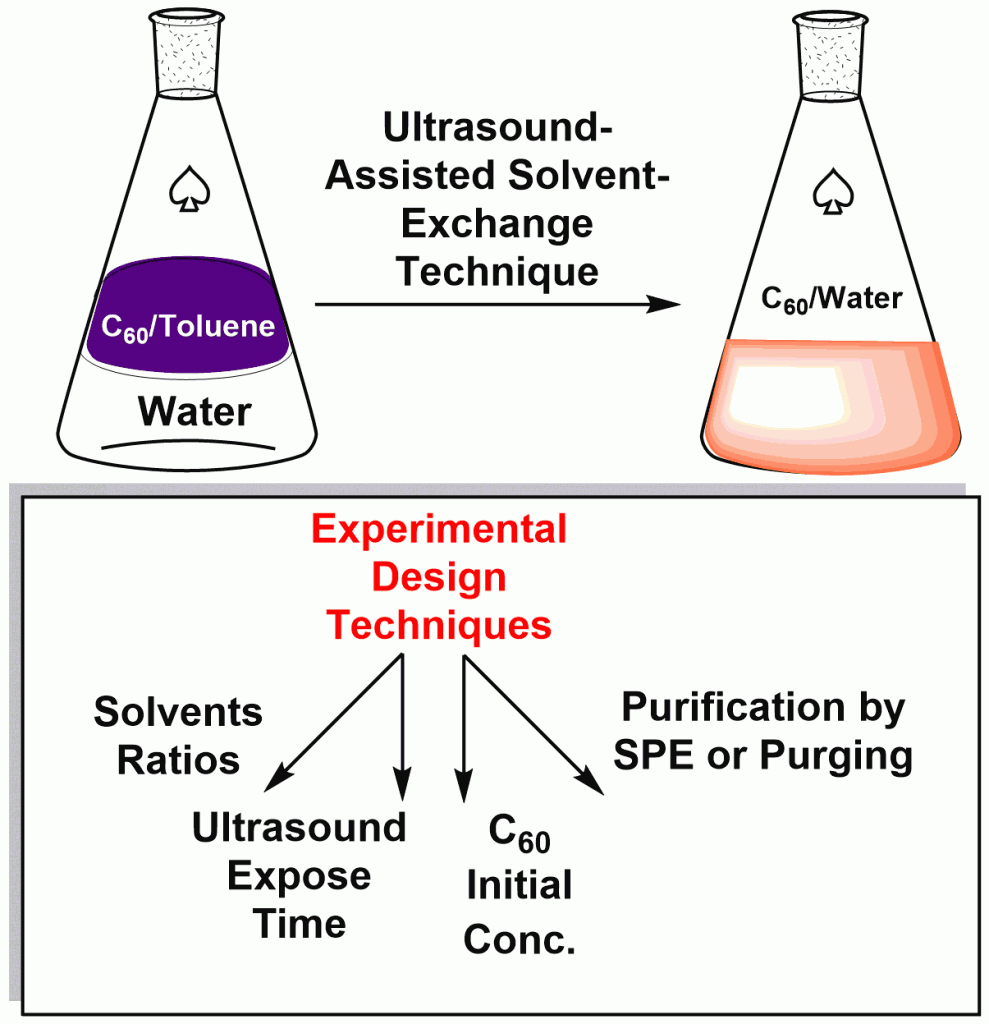
The ultrasound-assisted solvent-exchange technique for aqueous fullerene dispersions (AFD) of C60 (10-4 – 10-6 M) have been improved for high-yield synthesis, thereby achieving AFDs with total recovery over 90 %. Using ICP-AES, HPL-CUV, HGC-MS, the elemental and residual organic compounds have been estimated as not exceeding 3 ppm. The possible structure of fullerene clusters in AFD was assumed as {n[C60]mC6H5COO-(m – x)Na+}xNa+.
Keywords: solvent-exchange process, fullerene C60, stability, atomic spectroscopy.
PACS 68.55.ap, 81.05.ub
DOI 10.17586/2220-8054-2018-9-1-41-45