NANOSYSTEMS: PHYSICS, CHEMISTRY, MATHEMATICS, 2018, 9 (1), P. 49–51
Interactions of nanodiamonds and surfactants in aqueous suspensions
A. M. Vervald – Physical Department, M.V. Lomonosov Moscow State University, Moscow, Russia; alexey.vervald@physics.msu.ru
S. A. Burikov – Physical Department, M.V. Lomonosov Moscow State University, Moscow, Russia
I. I. Vlasov – A. M. Prokhorov General Physics Institute, Russian Academy of Sciences, Moscow, Russia
O. A. Shenderova – Adamas Nanotechnologies, Inc., Raleigh, NC, USA
T. A. Dolenko – Physical Department, M.V. Lomonosov Moscow State University, Moscow, Russia
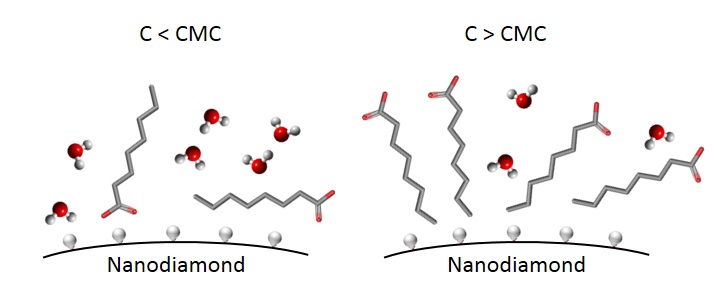
In this study, with the use of laser Raman spectroscopy the significant difference in intermolecular interactions of detonation nanodiamonds with hydrophilic and hydrophobic surface groups in aqueous solution of surfactants was observed. It was found that at low concentrations of sodium octanoate (before the micelle formation) the weakening of hydrogen bonds by nanodiamonds has a different dynamics for hydrophobic and hydrophilic nanodiamonds. However, with the addition of surfactants, this effect gradually decreases for both types of nanodiamonds and ends after the formation of micelles. Such effects are explained by the “shielding” effect of surfactant molecules surrounding nanodiamond particles.
Keywords: nanodiamonds, surfactants, suspensions, hydrogen bonds, dispersibility.
PACS 61.46.-w, 82.30.Rs, 82.70.Uv
DOI 10.17586/2220-8054-2018-9-1-49-51