NANOSYSTEMS: PHYSICS, CHEMISTRY, MATHEMATICS, 2018, 9 (5), P. 669–675
Electrocatalytic properties of ϒ-NiOOH nanolayers, synthesized by successive ionic layer deposition, during the oxygen evolution reaction upon water splitting in the alkaline medium
A. A. Lobinsky – Institute of Chemistry, Saint Petersburg State University, 26 University Pr., St. Peterhof, Saint Petersburg, 198504, Russia; lobinsky.a@gmail.com
V. P. Tolstoy – Institute of Chemistry, Saint Petersburg State University, 26 University Pr., St. Peterhof, Saint Petersburg, 198504, Russia; v.tolstoy@spbu.ru
I. A. Kodinzev – Institute of Chemistry, Saint Petersburg State University, 26 University Pr., St. Peterhof, Saint Petersburg, 198504, Russia; i.a.kod@mail.ru
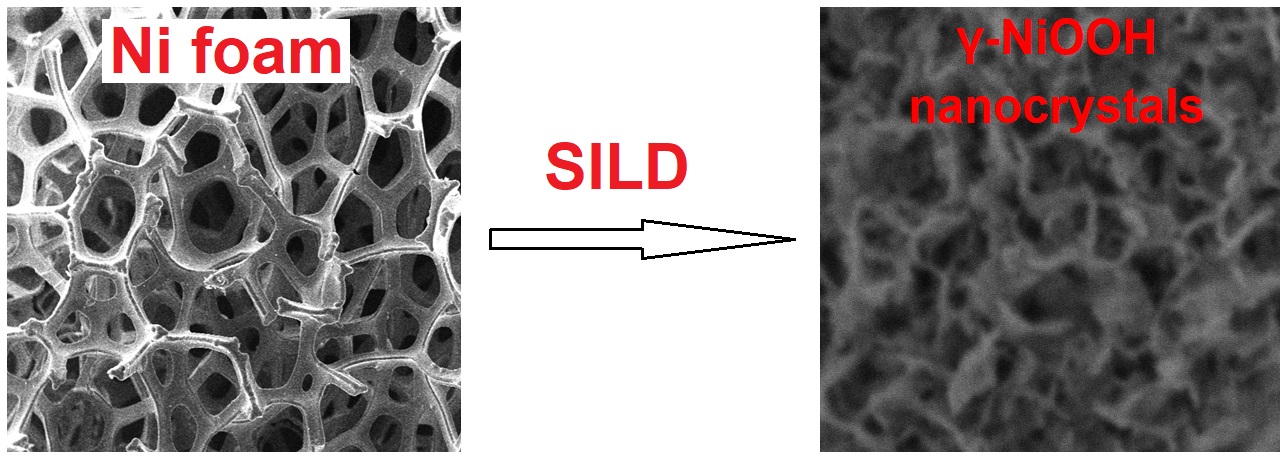
Nickel oxyhydroxide nanolayers were synthesized on the surface of nickel foam and single crystalline silicon through Successive Ionic Layer Deposition (SILD) method by using aqueous solutions NiSO4 and K2S2O8. The obtained nanolayers were characterized by SEM, XRD, FTIR and XPS spectroscopy. The electrochemical properties of the electrodes were defined from polarization curves. SEM images revealed that nanolayers are formed by nanosheets with a thickness of 6 – 10 nm. The nanolayers were shown to exhibit electrocatalytic properties during the oxygen evolution reaction upon water splitting in the alkaline medium. By setting the number of SILD cycles, these properties can be changed precisely. For a number of samples, synthesized after 30 – 120 SILD cycles, it was found that in the oxygen evolution reaction the lowest overpotential value of 260 mV and the lowest Tafel slope of 54 mV/dec are achieved for the sample, synthesized after 90 SILD cycles.
Keywords: NiOOH, nanolayers, SILD, oxygen evolution reaction.
PACS 82.65+r
DOI 10.17586/2220-8054-2018-9-5-669-675