NANOSYSTEMS: PHYSICS, CHEMISTRY, MATHEMATICS, 2019, 10 (5), P. 573–578
Successive ionic layer deposition of Co-doped Cu(OH)2 nanorods as electrode material for electrocatalytic reforming of ethanol
I. A. Kodintsev – Ioffe Institute, 194021 Saint Petersburg, Russia; i.a.kod@mail.ru
K. D. Martinson – Ioffe Institute, 194021 Saint Petersburg, Russia
A. A. Lobinsky – Saint Petersburg State University, Peterhof, 198504 Saint Petersburg, Russia
V. I. Popkov – Ioffe Institute, 194021 Saint Petersburg, Russia
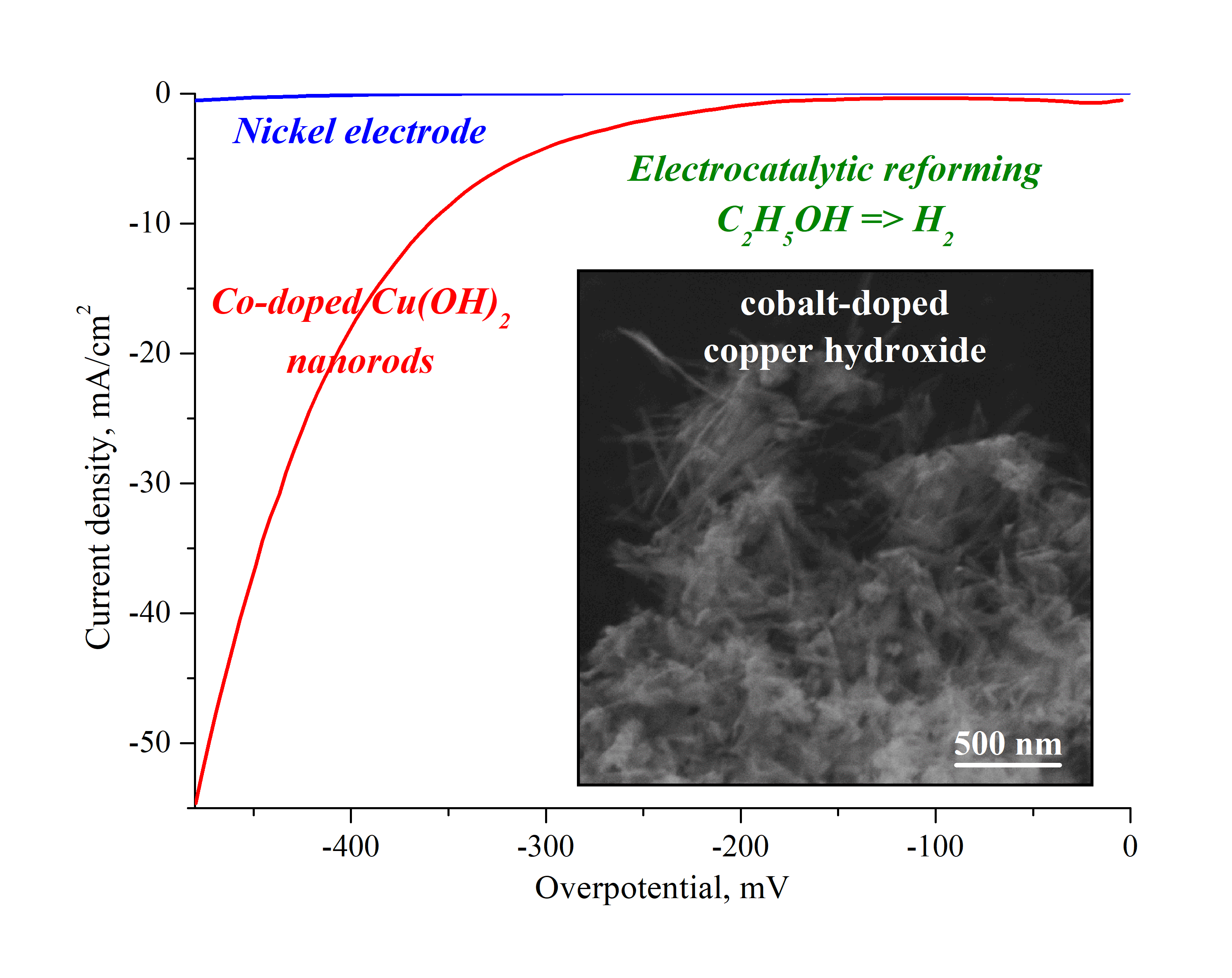
In this work, a facile and cost-effective layer by layer method was proposed to synthesize novel high stable and effective electrode material based on the Co-doped Cu(OH)2 nanocrystals. The crystals have orthorhombic structure and a rod-like morphology with a 23±2 nm in width and 43±4 nm in length. The composition of the nanocrystals corresponds to the 1 % Co-doped Cu(OH)2 by EDX with no noticeable impurities as it was found by FTIR spectroscopy. It was shown that nickel electrode modified with nanorods is characterized by an overvoltage value of -347 mV at 10 mA/cm2, which is 250 mV lower than that of an initial pure nickel electrode. The value of Tafel slope that reaches 138 mV/dec, high stability of the Co-doped Cu(OH)2 nanorods in chronopotentiometric (10 hours) and cyclic volamperometric (500 cycles) tests allows us to consider them as a prospective basis of electrode materials for the hydrogen evolution from renewable water-alcohol sources.
Keywords: copper hydroxide, nanocrystals, successive ionic layer deposition, hydrogen evolution, electrocatalytic reforming.
PACS 73.61.r, 81.15.z, 82.65.+r
DOI 10.17586/2220-8054-2019-10-5-573-578