NANOSYSTEMS: PHYSICS, CHEMISTRY, MATHEMATICS, 2020, 11 (4), P. 474–479
The effect of MgO additive on the g-C3N4 performance in electrochemical reforming of water-ethanol solution
M. I. Chebanenko – Ioffe Institute, St. Petersburg, 194021, Russia; m_chebanenko@list.ru
K. D. Martinson – Ioffe Institute, St. Petersburg, 194021, Russia
I.V. Matsukevich – Institute of General and Inorganic Chemistry of the National Academy of Sciences of Belarus, Minsk BY-220072, Republic of Belarus
V. I. Popkov – Ioffe Institute, St. Petersburg, 194021, Russia
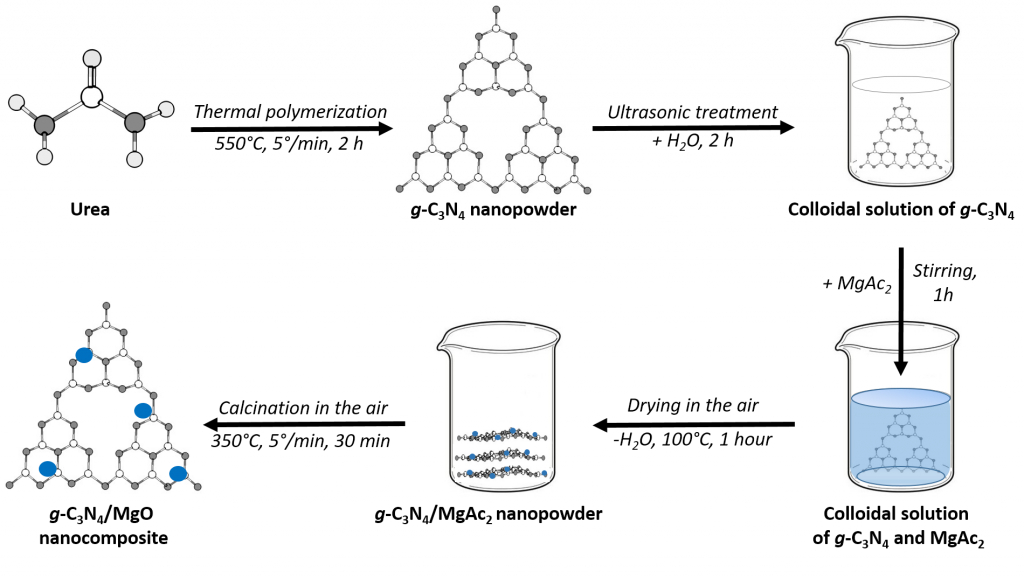
In this work, a simple wet-chemical route was proposed to synthesize g-C3N4/MgO (5% wt.) with enhanced electrocatalytic activity toward hydrogen evolution from water-ethanol (10% vol.) solution. It was found that synthesized nanocomposite is a single phase and chemically pure, consisting of graphitic carbon nitride (g-C3N4) and cubic magnesium oxide (MgO, periclase) with an average crystallite size of 15.5 nm and 9.5 nm, respectively. It was shown that magnesia nanoparticles are evenly distributed on the surface of g-C3N4 nanosheets and uniform distribution of components is observed over the nanocomposite volume. It was found that this feature leads to an improvement in the electrocatalytic characteristics of the synthesized nanocomposite. So, the g-C3N4/MgO-coated electrode has an overpotential of -251 mV, which is better than for a g-C3N4-coated (-264 mV) or pure nickel (-293 mV) electrode. Moreover, the nanocomposite-based electrode posses a low Tafel slope (-106.7 mV/dec) and high cyclic and chronopotentiometry stability.
Keywords: graphitic carbon nitride, magnesia, nanopowders, electrocatalytic reforming, hydrogen evolution reaction.
PACS 82.45.Yz
DOI 10.17586/2220-8054-2020-11-4-474-479