NANOSYSTEMS: PHYSICS, CHEMISTRY, MATHEMATICS, 2020, 11 (5), P. 529–536
A facile low-temperature deposition of Sn-rich tin (II) monosulfide colloid particles
N. S. Kozhevnikova – Institute of Solid State Chemistry of Ural Branch of the Russian Academy of Sciences, Pervomayskaya, 91, Ekaterinburg, 620990, Russia; kozhevnikova@ihim.uran.ru
L. N. Maskaeva – Ural Federal University named B. N. Yeltsin, Mira, 9, Ekaterinburg, 620002; Ural Institute of State Fire Service of EMERCOM of Russia, Mira str. 22, 620062, Ekaterinburg; larisamaskaeva@yandex.ru
E. E. Lekomtseva – Ural Federal University named B. N. Yeltsin, Mira, 9, Ekaterinburg, 620002, Russia; danserkatya13@mail.ru
L. A. Pasechnik – Institute of Solid State Chemistry of Ural Branch of the Russian Academy of Sciences, Pervomayskaya, 91, Ekaterinburg, 620990, Russia; pasechnik@ihim.uran.ru
A.Yu. Chufarov – Institute of Solid State Chemistry of Ural Branch of the Russian Academy of Sciences, Pervomayskaya, 91, Ekaterinburg, 620990, Russia; circulchufa@gmail.com
O. A. Lipina – Institute of Solid State Chemistry of Ural Branch of the Russian Academy of Sciences, Pervomayskaya, 91, Ekaterinburg, 620990, Russia; lipinaolgaa@yandex.ru
A. N. Enyashin – Institute of Solid State Chemistry of Ural Branch of the Russian Academy of Sciences, Pervomayskaya, 91, Ekaterinburg, 620990, Russia; enyashin@ihim.uran.ru
V. F. Markov – Ural Federal University named B. N. Yeltsin, Mira, 9, Ekaterinburg, 620002; Ural Institute of State Fire Service of EMERCOM of Russia, Mira str. 22, 620062, Ekaterinburg; v.f.markov@urfu.ru
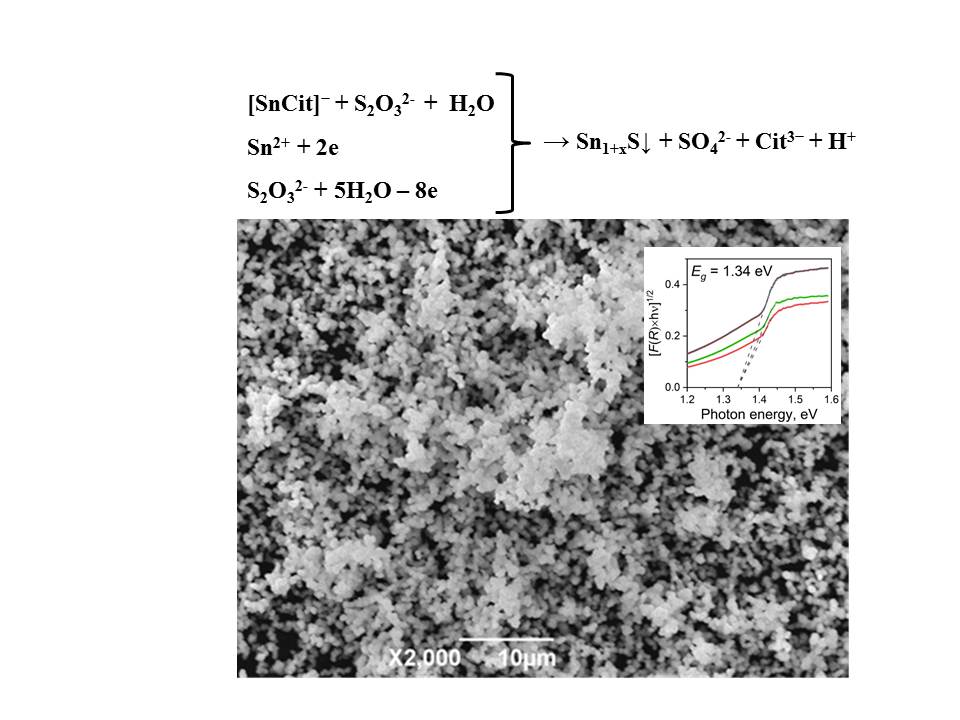
A novel, eco-friendly and low temperature synthesis of tin (II) monosulfide colloid particles is described. Chemical bath deposition was successfully applied for the deposition of polynanocrystalline SnS from acidic aqueous solutions. The characterization of the prepared samples was accomplished through elemental analysis, scanning electron microscopy, X-ray powder diffraction, and optical spectroscopy. The composition of tin (II) monosulfide colloids assembled of nanoparticles was found to be Sn-rich. Several simple scenarios for Sn surplus within SnS lattice (Svacancies at S-sublattice, Sn-atoms intercalated between SnS layers and Sn-doping of S-sites) have been analyzed by means of quantum chemical calculations. The potential application of the Sn1+xS colloid particles in solar cells as absorber material and as photocatalyst was demonstrated by measuring the optical properties.
Keywords: tin (II) sulfide, chemical bath deposition, optical band gap, Sn-rich.
PACS 81.10.Dn, 82.60.Lf, 82.70.Dd, 81.05.Hd
DOI 10.17586/2220-8054-2020-11-5-529-536