Nanosystems: Phys. Chem. Math., 2024, 15 (4), 510–519
Composite sorbent based on Fe3O4 with Fe(N2H4)xCly for the removal of Chromium(VI) from wastewater
Denis P. Ordinartsev – Institute of Metallurgy of the Ural Branch of the Russian Academy of Sciences, Yekaterinburg, Russia; denis_ordinartsev@mail.ru
Nadezhda V. Pechishcheva – Institute of Metallurgy of the Ural Branch of the Russian Academy of Sciences, Yekaterinburg; Ural Federal University named after the First President of Russia B. N. Yeltsin, Yekaterinburg, Russia; pechischeva@gmail.com
Svetlana Kh. Estemirova – Institute of Metallurgy of the Ural Branch of the Russian Academy of Sciences, Yekaterinburg, Russia; esveta100@mail.ru
Angelina V. Kim – Institute of Metallurgy of the Ural Branch of the Russian Academy of Sciences, Yekaterinburg; Ural Federal University named after the First President of Russia B. N. Yeltsin, Yekaterinburg, Russia; kim-angelina_95@mail.ru
Evgenii V. Sterkhov – Institute of Metallurgy of the Ural Branch of the Russian Academy of Sciences, Yekaterinburg, Russia; ev.sterhov@mail.ru
Sofia A. Petrova – Institute of Metallurgy of the Ural Branch of the Russian Academy of Sciences, Yekaterinburg, Russia; danaus@mail.ru
Galina A. Shilenko – Ural Federal University named after the First President of Russia B. N. Yeltsin, Yekaterinburg, Russia; g_shilenko@mail.ru
Corresponding author: Denis P. Ordinartsev, denis ordinartsev@mail.ru
DOI 10.17586/2220-8054-2024-15-4-510-519
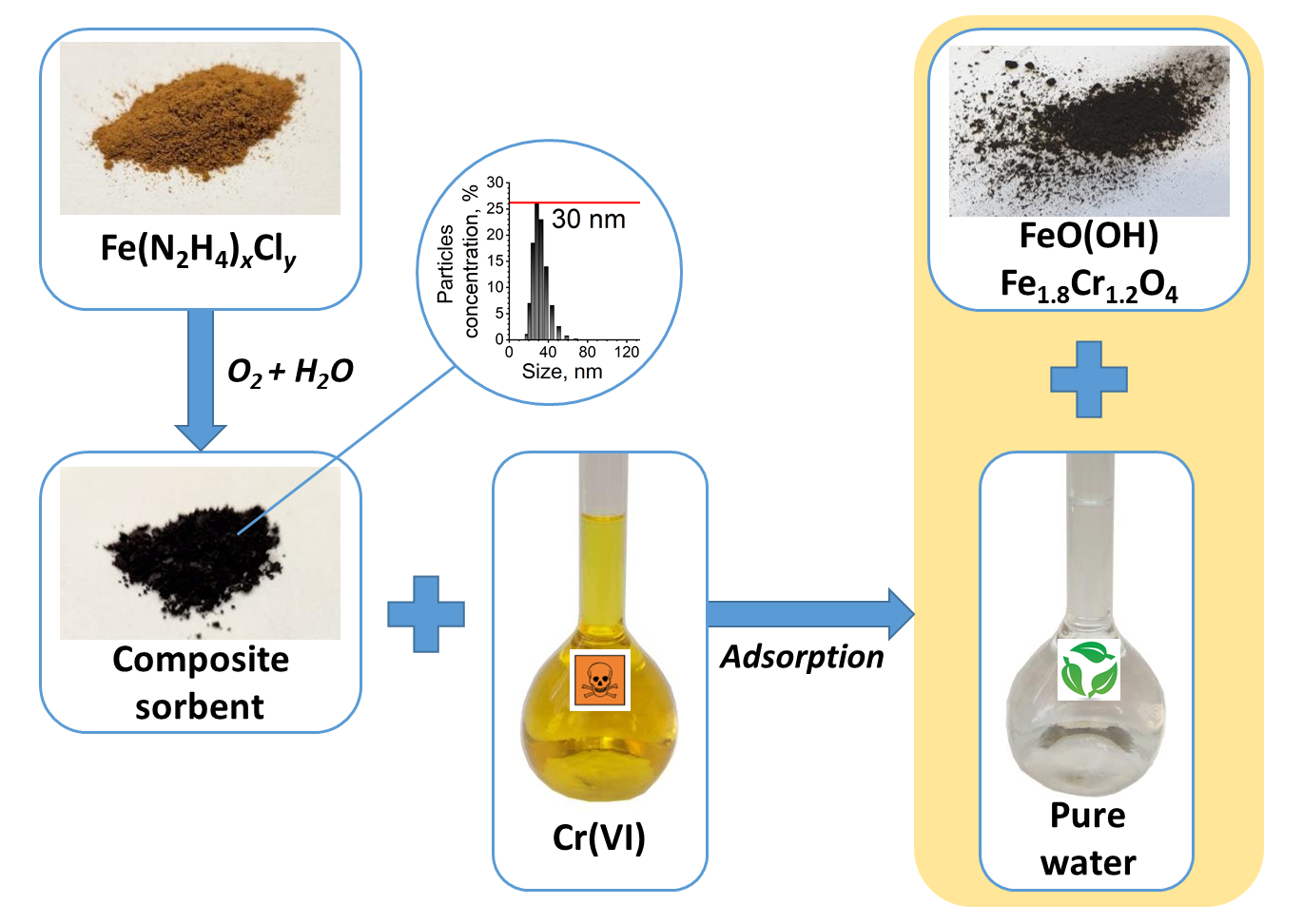
ABSTRACT The paper presents a methodology for the synthesis of iron complex with hydrazine hydrate Fe(N2H4)xCly. The Fe(N2H4)xCly complex was investigated by X-ray phase analysis and scanning electron microscopy. Upon hydrolysis, the Fe(N2H4)xCly complex forms a composite sorbent, which is Fe3O4 in a shell of Fe(N2H4)xCly complex. The composite sorbent can be used to treat wastewater from Cr(VI) ions and is effective in the pH range of 2 to 12. Based on the adsorption and electrokinetic potential data, a conclusion about the nature of the terminal groups of the adsorbent was made, a scheme of the structure of its electrical double layer and the adsorption mechanism were proposed. Depending on the conditions, Cr(VI) can be adsorbed on the composite sorbent or reduced to Cr(III). The efficiency of the composite sorbent in the removal of Cr(VI) ions was tested on a sample of real wastewater.
KEYWORDS Iron complex with hydrazine, magnetite sol, chromium adsorption, heavy metals, adsorption isotherms.
ACKNOWLEDGEMENTS The work was carried out according to the state assignment of IMET UB RAS using equipment of Collaborative usage centre “Ural-M” and with the support of the Ministry of Science and Higher Education of the Russian Federation (Ural Federal University Program of Development within the Priority-2030 Program), project No. 122080100074-1.
FOR CITATION Ordinartsev D.P., Pechishcheva N.V., Estemirova S.Kh., Kim A.V., Sterkhov E.V., Petrova S.A., Shilenko G.A. Composite sorbent based on Fe3O4 with Fe(N2H4)xCly for the removal of Chromium(VI) from wastewater. Nanosystems: Phys. Chem. Math., 2024, 15 (4), 510–519.
[In Russian] Д.П. Ординарцев, Н.В. Печищева, С.Х. Эстемирова, А.В. Ким, Е.В. Стерхов, С.А. Петрова, Г.А. Шиленко
Композитный сорбент на основе Fe3O4 с оболочкой Fe(N2H4)xCly для удаления хрома (VI) из сточной воды
УДК 66.021.2.081.3
АННОТАЦИЯ В работе представлена методика синтеза комплекса железа с гидратом гидразина Fe(N2H4)xCly. Комплекс Fe(N2H4)xCly исследован методами рентгенофазового анализа и сканирующей электронной микроскопии. При гидролизе комплекс Fe(N2H4)xCly образует композитный сорбент, который представляет собой Fe3O4 в оболочке из комплекса Fe(N2H4)xCly. Композитный сорбент может быть использован для очистки сточной воды от ионов Cr(VI) и эффективен в диапазоне рН от 2 до 12. На основе полученных данных по адсорбции и электрокинетического потенциала сделан вывод о характере терминальных групп адсорбента, предложена схема строения его двойного электрического слоя и механизм адсорбции. В зависимости от условий Cr(VI) может адсорбироваться на композитном сорбенте или восстанавливаться до Cr(III). Эффективность композитного сорбента при удалении ионов Cr(VI) проверена на образце реальной сточной воды.
КЛЮЧЕВЫЕ СЛОВА комплекс железа с гидразином, золь магнетита, адсорбция хрома, тяжелые металлы, изотермы адсорбции