Nanosystems: Phys. Chem. Math., 2023, 14 (1), 112–119
Synthesis and thermal behavior of KCe2(PO4)3, a new full-member in the AIMIV2 (PO4)3 family
Taisiya O. Kozlova – Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences, Moscow, Russia; taisia.shekunova@yandex.ru
Darya N. Vasilyeva – Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences, Moscow; National Research University Higher School of Economics, Moscow, Russia; dnvasileva_1@edu.hse.ru
Daniil A. Kozlov – Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences, Moscow; Lomonosov Moscow State University, Moscow, Russia; kozlov@inorg.chem.msu.ru
Mariia A. Teplonogova – Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences, Moscow; Lomonosov Moscow State University, Moscow, Russia; m.teplonogova@gmail.com
Alexander E. Baranchikov – Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences, Moscow, Russia; a.baranchikov@yandex.ru
Nikolay P. Simonenko – Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences, Moscow, Russia; n_simonenko@mail.ru
Vladimir K. Ivanov – Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences, Moscow, Russia; van@igic.ras.ru
Corresponding author: T.O. Kozlova, taisia.shekunova@yandex.ru
PACS 65.40.-b; 61.50.-f; 61.66.Fn
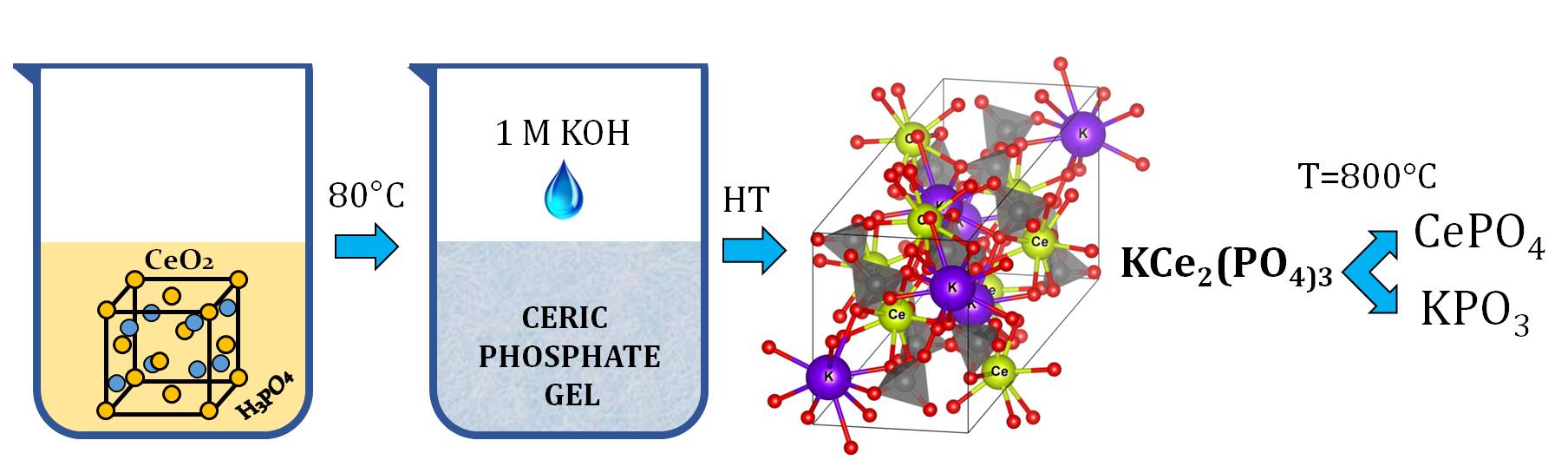
ABSTRACT Hydrothermal treatment of nanoscale amorphous ceric phosphate gel in KOH aqueous solutions was found to result in a new KCe2(PO4)3 phase. The refinement of the KCe2(PO4)3 structure showed that it was isostructural to recently reported (NH4)Ce2(PO4)3. For the KCe2(PO4)3 phase, the unit cell parameters (sp. gr. C2/c) were a = 17.3781(3) Å, b = 6.7287(1) Å, c = 7.9711(2) Å, β = 102.351(1) °, V = 910.53(4) Å3, Z = 4. The thermal decomposition of KCe2(PO4)3 at 800 °C resulted in the mixture of crystalline CePO4 and KPO3.
KEYWORDS cerium, potassium, polyphosphate, channel, hydrothermal
ACKNOWLEDGEMENTS This work was supported by Russian Science Foundation (Grant no. 21-73-00294, https://rscf.ru/en/project/21-73-00294/) using the equipment of the JRC PMR IGIC RAS. The authors thank Dr. A. V. Gavrikov for FT-IR spectroscopy studies.
FOR CITATION Kozlova T.O., Vasilyeva D.N., Kozlov D.A., Teplonogova M.A., Baranchikov A.E., Simonenko N.P., Ivanov V.K. Synthesis and thermal behavior of KCe2(PO4)3, a new full-member in the AIMIV2 (PO4)3 family. Nanosystems: Phys. Chem. Math., 2023, 14 (1), 112–119.
[In Russian] Козлова Т.О., Васильева Д.Н., Козлов Д.А., Теплоногова М.А., Баранчиков А.Е., Симоненко Н.П., Иванов В.К.
Синтез и термическое поведение KCe2(PO4)3 — нового соединения в семействе AIMIV2 (PO4)3
АННОТАЦИЯ Гидротермальной обработкой наноразмерного аморфного церийфосфатного геля в водном растворе KOH была получена новая фаза KCe2(PO4)3. Уточнение структуры KCe2(PO4)3 показало, что она изоструктурна с ранее описанной фазой (NH4)Ce2(PO4)3. Параметры элементарной ячейки KCe2(PO4)3 (пр. гр. C2/c) составили a = 17.3781(3) Å, b = 6.7287(1) Å, c = 7.9711(2) Å, β = 102.351(1) °, V = 910.53(4) Å3, Z = 4. Термическое разложение KCe2(PO4)3 при 800°C привело к образованию смеси кристаллических CePO4 и KPO3.
КЛЮЧЕВЫЕ СЛОВА церий; калий; полифосфат; канал; гидротермальный