NANOSYSTEMS: PHYSICS, CHEMISTRY, MATHEMATICS, 2018, 9 (4), P. 558–567
High-temperature synthesis of finely dispersed oxide materials and C12A7:e electrides in carbon nanoreactor conditions
A. M. Volodin – Boreskov Institute of Catalysis, Prospekt Lavrentieva, 5, Novosibirsk, 630090, Russia; volodin@catalysis.ru
A. F. Bedilo – Boreskov Institute of Catalysis, Prospekt Lavrentieva, 5, Novosibirsk, 630090, Russia
V. O. Stoyanovskii – Boreskov Institute of Catalysis, Prospekt Lavrentieva, 5, Novosibirsk, 630090, Russia
V. I. Zaikovskii – Boreskov Institute of Catalysis, Prospekt Lavrentieva, 5, Novosibirsk, 630090, Russia
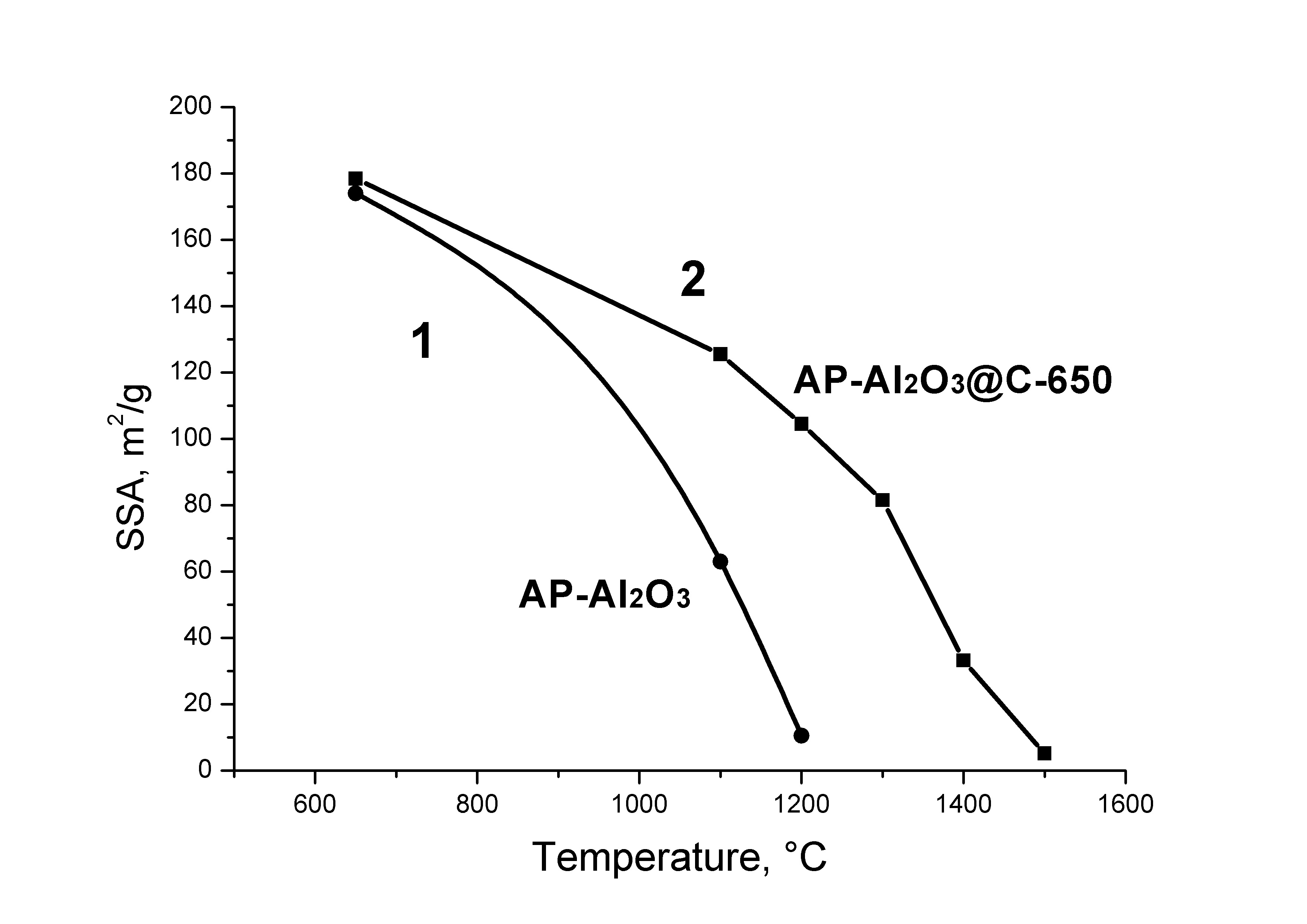
Solid-state transformations of the oxide core in core-shell structures Oxide@C consisting of oxide nanoparticles covered with a carbon coating were studied at temperatures of up to 1500 °C. It is shown that such coating can stabilize the size of the oxide core nanoparticles for alumina, zirconia, calcium and lanthanum aluminates and act as a shell of a nanoreactor where phase and chemical transformation can take place. For ZrO2@C and δ-Al2O3@C it is demonstrated that it is the preservation of the small particle size that accounts for the preservation of cubic ZrO2 and δ-Al2O3 until the carbothermal reduction temperatures of the corresponding oxides (above 1400 °C for Al2O3). The electride state C12A7:e is shown to be formed in C12A7@C material at temperatures above its melting point. The surface of activated C12A7 was found to have a significant concentration of active OH radicals capable of converting diphenylamine into stable nitroxyl radicals.
Keywords: Core-shell, nanocrystalline oxide, electride, ZrO2, Al2O3, carbon nanoreactor.
DOI 10.17586/2220-8054-2018-9-4-558-567